What is Non-Compartmental Analysis?
Non-compartmental analysis, also known by its acronym NCA, is a simple and descriptive methodology used to understand the pharmacokinetic properties of a drug. It is an a posteriori analysis in that pharmacokinetic metrics are determined using actual data (i.e., measured serial drug concentrations over time in a clinical study).
The NCA methodology assumes that the body behaves like a single compartment and does not make assumptions about hypothetical body compartments. This means that the drug is evenly distributed throughout the body with no difference in the rate of elimination from different organs. The NCA methods generally involve the use of algebraic equations to estimate pharmacokinetic metrics that help describe a drug’s absorption, distribution, metabolism and elimination from the body.
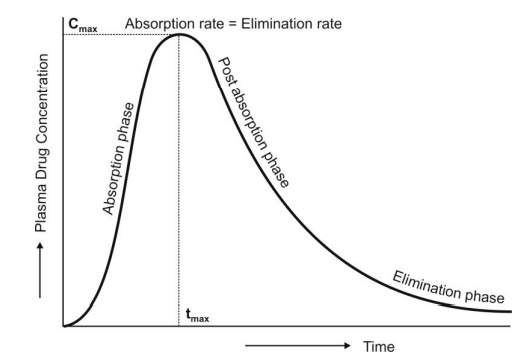
The common pharmacokinetic metrics obtained from a NCA include, the: maximum drug concentration achieved (Cmax), time to achieve the maximum drug concentration (Tmax), area under the concentration-time curve (AUC), terminal elimination half-life (t1/2), trough concentration (Ctrough) and clearance (CL).
What can we learn from the pharmacokinetic metrics obtained from a NCA?
What is Cmax?
Cmax is the maximum drug concentration achieved in a specified matrix, such as blood or plasma, after a drug has been administered prior to a second/subsequent dose being administered. The numerical value of Cmax does not describe anything related to the distribution of the drug, as drug concentrations may be vary significantly in different organs. The Cmax following an intravenous dose of drug is dependent on the study protocol. Whilst, the Cmax following an extravenous dose of a drug is dependent on the extent and rate of drug absorption and disposition. The magnitude of Cmax is of particular importance in guiding dose selection when it is near the upper therapeutic limit as this is when potential adverse events may occur.
What is Tmax?
Tmax is the time taken to achieve Cmax after a drug has been administered. Tmax is governed by the rate of drug absorption and elimination. It occurs when the rate of absorption is equal to the rate of elimination. The value of Tmax may be of clinical interest when the time to effect is driven by Cmax.
What is AUC?
AUC is a measure of the total drug exposure in the body over time. AUC is a key metric for evaluating drug efficacy and safety as it provides insight into the extent of drug exposure and rate of clearance from the body. AUC may be used in therapeutic drug monitoring of drugs with a narrow therapeutic window, or, when wanting to determine whether two formulations of the same drug provide the same drug exposure.
What is half-life?
Half-life (t1/2) is the time it takes for the concentration or amount of drug in the body to reduce by half (50%). It provides insight into the duration of action for a drug and may be used to guide dosing regimens/schedules. For instance, drugs with a short half-life may be administered more frequently in order to maintain a therapeutic concentration required for an effect. Whilst, drugs with a longer half-life may be given less frequently.
What is Ctrough?
Ctrough is the concentration achieved in a specified matrix immediately prior to the administration of a subsequent dose. The Ctrough may be considered in clinical practice to ensure that concentrations are above the minimum concentration limit to ensure therapeutic efficacy. The Ctrough may be different to Cmin which is the minimum concentration achieved during the interval of two drug doses being administered.
What is Clearance?
Clearance describes the ability of the body to eliminate a substance, such as a drug, from the body without identifying the specific underlying removal mechanisms. It is defined as the volume of a specified matrix (e.g. plasma) from which a substance is completely removed per unit time. Clearance is important as it is used to guide dosing regimens in order to achieve a specific drug concentration.